Tetranuclear Titanium Nitride Cluster: Dintrogen Cleavage under Mild Conditions and Its Reactivity
Hanhua Xu, Hanjie Wu, Xiao Chen, Ze-jie Lv, Zhongzhen Wang, Zhenfeng Xi, Junnian Wei*
Angew. Chem. Int. Ed., 2025. DOI: 10.1002/anie.202505312.
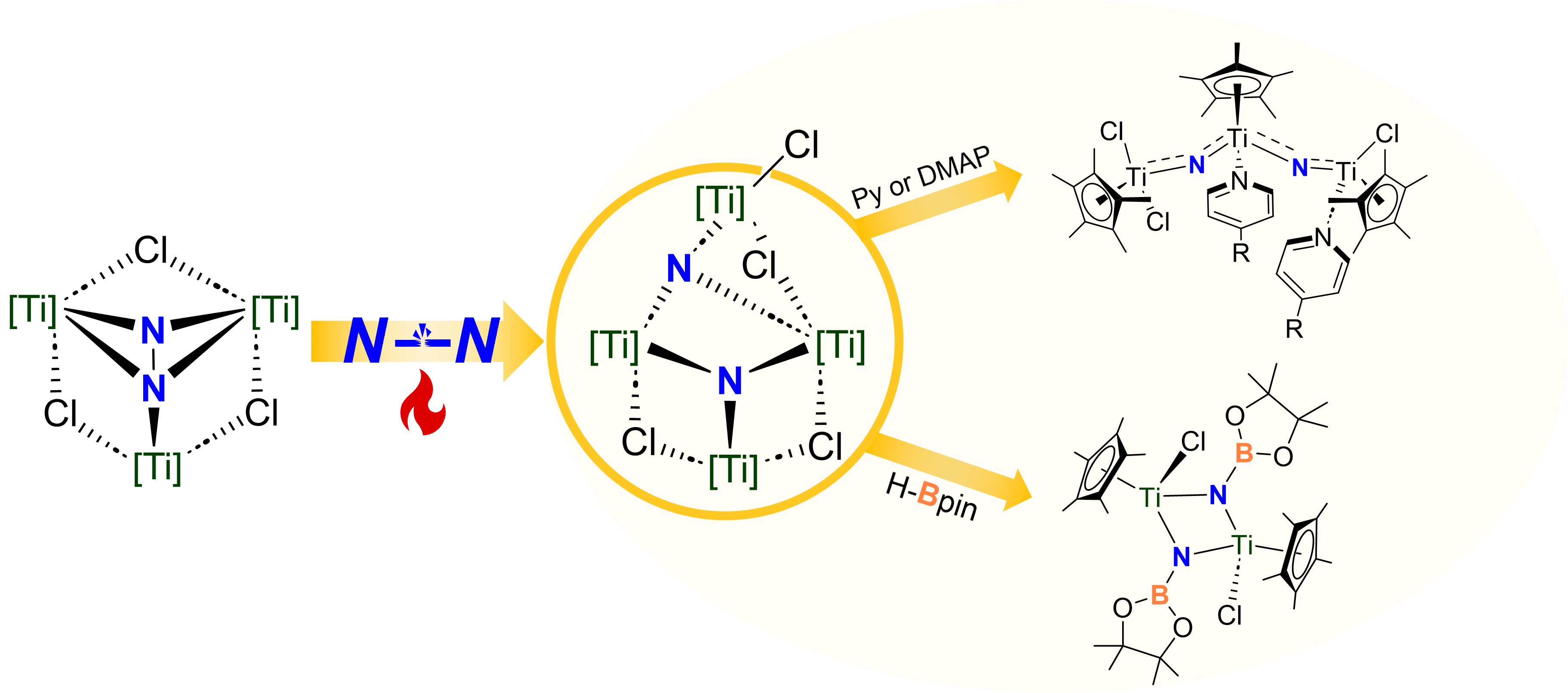
Heating the readily available trinuclear Ti dinitrogen complex [{TiCp*(μ-Cl)}3(μ3-η1: η2: η2-N2)] 1(Cp* = η5-C5Me5), originally reported by C. Yélamos and J. Jover, in benzene at 80 °C leads to the cleavage of N≡N bond, forming a tetranuclear titanium nitride cluster [{TiCp*}4Cl(μ-Cl)3(μ3-N)2] 2. The structure of 2 was confirmed by single-crystal X-ray diffraction analysis. Kinetics study and DFT calculations indicate that the energy barrier for the NN bond cleavage in the transformation from 1 to 2 is about 26 kcal/mol. Complex 2 exhibits reactivity toward various electrophiles, producing a range of nitrogen-containing compounds in the presence of air, while concurrently regenerating Ti(IV) salts. Furthermore, complex 2 reacts with pinacolborane, leading to the formation of N-B bond derivatives [{TiCp*(μ-NBPin)Cl}2] 3. When complex 2-15N is treated with pyridine or 4-dimethylaminopyridine (DMAP), it dissociates to form a bent trinuclear Ti nitrido complex [{TiCp*}3Cl3(L)2(μ-15N)2] 4-L (L = py or DMAP).